DESCRIPTION
Amino Acid Sequence
General description
Application
Amyloid β Protein Fragment 1-40 has been used:
- within the temperature based mostly conformational research utilizing Fourier remodel infrared/differential scanning calorimetry (FT-IR/DSC) research
- as a reference normal in sandwich-type enzyme immunoassay for quantifying amyloid A4 protein in cerebrospinal fluid of sufferers with head trauma
- as a part of embryonic stem cell medium to inhibit amyloid deposition in fibroblasts
Biochem/physiol Actions
Reconstitution
Other Notes
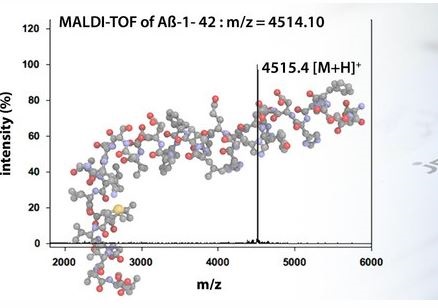
amyloid
Abstract
Alzheimer’s illness (AD) pathogenesis is broadly believed to be pushed by the manufacturing and deposition of the β-amyloid peptide (Aβ). For a few years, investigators have been puzzled by the weak to nonexistent correlation between the quantity of neuritic plaque pathology within the human mind and the diploma of scientific dementia. Recent advances in our understanding of the event of amyloid pathology have helped remedy this thriller. Substantial proof now signifies that the solubility of Aβ, and the amount of Aβ in numerous swimming pools, could also be extra carefully associated to illness state. The composition of those swimming pools of Aβ displays completely different populations of amyloid deposits, and has particular correlates with the scientific standing of the affected person. Imaging applied sciences, together with new amyloid imaging brokers based mostly on the chemical construction of histologic dyes, at the moment are making it potential to trace amyloid pathology together with illness development within the dwelling affected person. Interestingly, these approaches point out that the Aβ deposited in AD is completely different from that present in animal fashions. In basic, deposited Aβ is extra simply cleared from the mind in animal fashions, and doesn’t present the identical bodily and biochemical traits because the amyloid present in AD. This raises essential points relating to the event and testing of future therapeutic brokers.