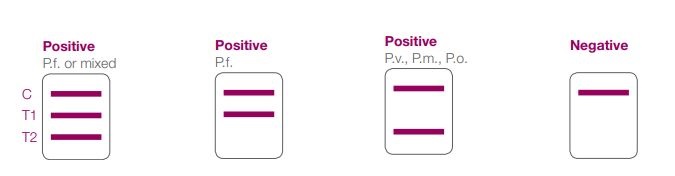
The BinaxNOW Malaria verify is a speedy and straightforward diagnostic verify which will
exactly differentiate a deadly P. falciparum an an infection from a pan-malarial
an an infection introduced on by P. vivax, P. ovale or P. malariae.
■ three straightforward steps
■ 1 reagent
■ Dependable Ends in 15 minutes
■ Sensitivity: 99.7% (P.f.); 93.5% (P.v.)*
■ Specificity: 94.2% (P.f.); 99.8% (P.v.)

binaxnow precise
Description
A speedy and straightforward diagnostic verify which will exactly differentiate a deadly P. falciparum an an infection from a pan-malarial an an infection introduced on by P. vivax, P. ovale, or P. malariae
- For differential evaluation of a malaria an an infection
- Simply three straightforward steps, using one reagent
- Dependable ends in 15 minutes
- CLIA Complexity: Average
- CPT Code: 87899
- Sensitivity: 99.7% (P.f.); 93.5% (P.v.)
- Specificity: 94.2% (P.f.); 99.8% (P.v.)
BNX665000: Malaria Check Equipment – World
BNX665025: Malaria Check Equipment – US
BNX665010: Optimistic Management
ABSTRACT
| Organism | Equipment title | Producer – distributora | Sort of Checkb |
|---|---|---|---|
| Plasmodium | BinaxNOW® Malaria Check | Inverness Medical | Fast (HRP2 and aldolase) |
| Malaria-Ag | Cellabs | EIA | |
| OptiMal | Movement | Fast (LDH) | |
| MAKROmed malaria verify | MAKROmed Manufacturing, LTD | Fast (HRP2) | |
| Paracheck Pf | Orchid | Fast (HRP2) | |
| Visitect Malaria Pf | Omega Diagnostics LTD | Fast (HRP2) | |
| Wuchereria bancrofti | ICT Filariasis | Binax | Fast |
| Filariasis Ag-CELISA | Cellabs | EIA |
Parasite lactate dehydrogenase (pLDH) is produced by asexual and sexual phases (gametocytes) of malaria parasites. Check kits that are presently obtainable detect pLDH from all four species of Plasmodium. They will distinguish P. falciparum from the non-falciparum species, nonetheless cannot distinguish between P. malariae, P. ovale, and P. vivax. Checks that detect pLDH do not generate persistent optimistic outcomes following chemotherapy, similar to the HRP-II verify.
binaxnow precise
At current, the one obtainable RDT for malaria in america is the BinaxNOW Malaria Check. The Binax assay contains every the HRP II (for P. falciparum) and aldolase which is a pan-malarial antigen. It cannot separate non-falciparum species. The Binax assay must on a regular basis be accompanied by thick and thin film microscopy.
Specs
| Average | |
| Antigens to all 4 species of malaria, Plasmodium vivax, Plasmodium ovale and Plasmodium malariae in complete blood | |
| 15 min. |
| 87899 | |
| Entire Blood |
[Linking template=”default” type=”products” search=”BinaxNOW Malaria” header=”3″ limit=”25″ start=”2″ showCatalogNumber=”true” showSize=”true” showSupplier=”true” showPrice=”true” showDescription=”true” showAdditionalInformation=”true” showImage=”true” showSchemaMarkup=”true” imageWidth=”” imageHeight=””]