In June 2020, JOYSBIO Biotechnology proudly launched a brand new COVID-19 Antigen Rapid Test Kit (Colloidal Gold). The new coronavirus antigen check equipment is a lateral circulation immunoassay for the qualitative detection of SARS-COV-2 antigen (nucleocapsid protein) in higher respiratory samples with nasal swabs or saliva in the course of the acute part of an infection. An uncut sheet format is obtainable.
Features
- 15-minute speedy detection
- Easy-to-operate coronavirus antigen check
- Less-invasive nasal (NS) swab pattern assortment
- CE-IVD marked
- Available in half of/5/20 checks/field
Performance Characteristics
JOYSBIO’s coronavirus Ag check equipment was independently evaluated at Centro Diagnostico Delta S.r.l. in Italy between October 2020 and January 2021. A complete of 107 constructive specimens had been examined with JOYSBIO’s COVID-19 Antigen Rapid Test Kit. These specimens had been collected from sufferers who’re suspected of COVID-19 with nasal swabs. The coronavirus antigen check equipment’s sensitivity and specificity are in contrast towards a CE-IVD marked RT-PCR check equipment. This scientific analysis is performed beneath the belief that SARS-CoV is not spreading locally.
According to the scientific evaluation of 492 samples, the detection sensitivity is 98.13%, and the specificity is 99.22%.
- Positive Percent Agreement (PPA) = 105/107 (98.13%) (95%CI: 93.4%~99.8%)
- Negative Percent Agreement (NPA) = 382/385 (99.22%) (95%CI:97.7%~99.8%)
- Accuracy = (105+382)/492×100%=98.98%
- Kappa = 2×(105×382-3×2)/(108×385+107 ×384) = 0.97>0.5
The restrict of detection (LOD) of this product is 1.6 x 102 TCID50/mL, calculated by way of a gradient dilution methodology.
sars-cov-2-rapid-antigen-test
COVID-19 Antigen Rapid Test Principle
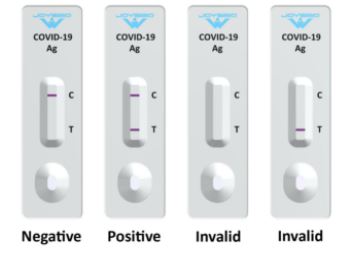
The coronavirus antigen speedy check equipment is a lateral circulation assay that qualitatively detects the presence of nucleocapsid (N) protein in higher respiratory samples (nasal swabs). This lateral circulation assay is designed with the sandwich immunoassay format. When the specimen is added onto the pattern pad of a check cassette, coronavirus N protein binds with colloidal gold-labeled SARS-CoV-2 N protein antibody to type an antibody-antigen (Ab-Ag) advanced. The Ab-Ag advanced is captured by SARS-CoV-2 N protein antibody (Rabbit monoclonal antibody) when migrating to the check line beneath capillary motion. A red-colored band will seem on the check line, which signifies the specimen is COVID-19 nucleocapsid protein constructive. No shade band will seem on the check line if the specimen doesn’t include any coronavirus antigen (N protein), or the antigen stage is under detection restrict.
- Twist off the cap of the buffer bottle, rigorously dispense all buffer into the extraction tube。
- After amassing higher respiratory pattern with nasal swab, insert the swab into the extraction tube, plunge the swab up and down within the fluid for at least 10 seconds. Hold the swab towards the underside of the tube, rotate three turns. DO NOT splash liquid out of the tube.
- Remove the swab whereas squeezing the edges of the tube to extract the liquid from the swab.
- Press the nozzle cap firmly onto the extraction tube. Mix completely by swirling or flicking the underside of the tube.
- Gently squeeze the tube’s inflexible physique, dispense two (2) drops of the buffer-specimen combination into the pattern effectively on the coronavirus antigen check cassette.
- Read the check outcomes between 15 and 20 minutes. Do not learn the outcomes after 20 minutes.
covid-19-antigen-rapid-test-kit
Stage 1: Nasopharyngeal swab specimen assortment:
1. Collect a nasopharyngeal swab specimen by inserting the sterile swab into the nostril.
2. Push the sterile swab till resistance is met on the stage of the turbinate.
3. Rotate the sterile swab a number of instances towards the nasopharyngeal wall & go away within the place for 10 seconds to saturate the swab tip.
4. Remove the swab from the nostril rigorously.
5. Place the swab specimen into the viral transport medium buffer tube and shut the tube tightly.
6. Transport the swab pattern in VTM to the laboratory in chilly chain.
7. The pattern may be saved within the Room temperature (Below 30◦C) as much as 24 hrs from the time of pattern assortment or at 2 – 8◦C for as much as 48 hrs from the time of pattern assortment.
Stage 2: Sample preparation for testing:
1. Sample preparation must be carried out in BSL-2 stage cupboard within the Laboratory.
2. Mix the swab specimen in VTM tube effectively (vortex roughly 3-5 seconds).
3. Transfer 100 μL VITROS® SARS-CoV-2 Antigen Extraction Buffer right into a labelled new pattern tube.
4. Add 400 μL viral pattern to the above tube (to take care of 1:Four ratio of extraction buffer: pattern)
5. Mix effectively (Cap/Plug the pattern tube and vortex roughly 3-5 seconds)
Stage 3: Sample processing in VITROS® programs:
1. Place/load the ready/extracted pattern tube in stage 2, after de-capping on to the VITROS® instrument V3600/V5600/XT7600; An quantity of 80 μL of extracted pattern is used for every willpower.
2. Program VITROS 3600 / VITROS 5600 / VITROS XT 7600 system to course of the samples for CV2Ag. The system can used to program check both in a STAT/Random/Batch mode.
3. System processes the samples robotically utilizing disposable VersaTips for each pattern in addition to reagents to forestall any cross-contamination. The outcomes shall be delivered in 48 minutes after pattern aspiration, within the type of S/Co worth.
[Linking template=”default” type=”products” search=”Antigen Rapid Test Kit” header=”3″ limit=”21″ start=”2″ showCatalogNumber=”true” showSize=”true” showSupplier=”true” showPrice=”true” showDescription=”true” showAdditionalInformation=”true” showImage=”true” showSchemaMarkup=”true” imageWidth=”” imageHeight=””]