INTENDED USE
The Clarity Pregnancy One Step Rapid Test (Cassette) is an in vitro diagnostic
visible qualitative immunochromatographic assay designed for the speedy
willpower of human chorionic gonadotropin (hCG) in urine to assist within the
detection of being pregnant. For skilled use and OTC self take a look at use.
SUMMARY AND EXPLANATION
Human chorionic gonadotropin (hCG) is a glycoprotein hormone produced in
being pregnant that’s made by the growing embryo after conception and later by the syncytiotrophoblast. In regular being pregnant, hCG could be detected in urine as early as 1-2 weeks after conception. hCG ranges proceed to rise very quickly, often exceeding 100 mIU/mL by the primary missed menstrual interval, and peaking within the 100,000-200,000 mIU/mL vary about 10-12 weeks into being pregnant. Early
being pregnant testing, on the whole, relies on the detection or measurement of hCG.
The Clarity Pregnancy One Step Rapid Test makes use of a mixture of monoclonal
and polyclonal antibodies to selectively detect elevated ranges of hCG in urine.
Positive specimens react with the particular antibody-hCG-colored conjugate to type a coloured line on the take a look at line area of the membrane. Absence of this coloured line suggests a detrimental end result. At the extent of claimed sensitivity, the Clarity Pregnancy One Step Rapid Test exhibits no cross-reactivity interference from the structurally associated glycoprotein hormones hFSH, hLH and hTSH at excessive physiological ranges.
PRINCIPLE OF THE TEST
Human chorionic gonadotropin (hCG) is a hormone, produced by the growing
placenta shortly after the conception and secreted into the urine. The being pregnant
take a look at incorporates antibodies which particularly react with this hormone.
The capillary motion carries the specimen emigrate alongside the membrane. When hCG within the pattern reaches the Test Zone area of the membrane, it would type a coloured line. The absence of this coloured line suggests a detrimental end result.
To function a process management, a coloured line will seem on the management zone area, if the take a look at has been carried out correctly.
MATERIALS SUPPLIED
1. One Pregnancy Test pouch, containing a cassette, dropper, and desiccant. The
desiccant is for storage functions solely and isn’t used within the take a look at procedures.
2. Instructions to be used.
REAGENTS
Coated Antibodies: Control area: Goat anti-mouse (IgG) polyclonal antibody
Test area: Mouse monoclonal anti-hCG antibody
A Labeled Antibodies: The colloidal gold conjugate of monoclonal anti-hCG antibody B
WARNINGS AND PRECAUTIONS
1. This take a look at is designed for “in vitro diagnostic” use.
2. Read directions rigorously earlier than utilizing this take a look at.
3. This package is for exterior use solely. Do not swallow.
4. Do not use the take a look at package past the expiration date.
5. Do not use the package if the pouch is punctured or shouldn’t be effectively sealed.
6. Keep out of the attain of youngsters.
7. Urine specimens could also be infectious; Insure correct handing and discard all used
gadgets in keeping with native laws.
8. The take a look at is for single use solely. Do not reuse it.
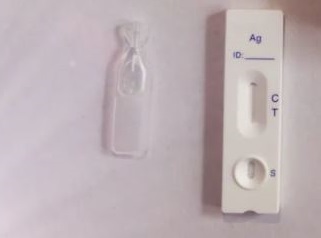
One Step Rapid Test package
COLLECTION AND STORAGE OF SPECIMENS
Any urine specimen is suitable for being pregnant testing however the first morning urine specimen is perfect due to its highest focus of HCG. Urine ought to be collected in a clear container previous to testing.
READING TEST RESULTS
Positive (Pregnant)
Two distinct purple strains seem. One line ought to be within the management area (C) and
one other line ought to be within the take a look at area (T). This means you might be most likely pregnant. Negative (Not pregnant) One purple line seems within the management area (C).No obvious purple or pink line seems within the take a look at area (T).This means you might be most likely not pregnant.
Invalid The result’s invalid if no purple line seems within the management area (C), even when a line seems within the take a look at area (T). You ought to repeat the take a look at with a brand new strip.
NOTE: if the take a look at line is weak, it’s endorsed that the take a look at be repeated in 48
hours.
QUALITY CONTROL
Internal procedural controls are included within the take a look at. A coloured band showing within the management area (C) is taken into account an inner optimistic procedural management, confirming adequate specimen quantity and proper procedural method.
External controls should not equipped with this package. It is beneficial that optimistic and detrimental controls be examined as a very good laboratory follow to verify the take a look at process and to confirm correct take a look at efficiency.
[Linking template=”default” type=”products” search=”One Step Diagnostic Rapid” header=”2″ limit=”28″ start=”1″ showCatalogNumber=”true” showSize=”true” showSupplier=”true” showPrice=”true” showDescription=”true” showAdditionalInformation=”true” showImage=”true” showSchemaMarkup=”true” imageWidth=”” imageHeight=””]