Please Note:
Below listed kits are validated with the talked about batch quantity solely. Responsibility for batch to batch consistency doesn’t lies with ICMR.
Minimum acceptance standards of sensitivity and specificity of Rapid Ag Test Kits:
Validated as a Point of Care Test (POCT) with out transport to a laboratory setupSensitivity: 50% and above; Specificity: 95% and above
Validated in a laboratory setup with samples collected in Viral Transport Medium (VTM)- Sensitivity: 70% and above; Specificity: 99% and above
Antigen based mostly rapid checks that are US-FDA authorized can be utilized instantly after due advertising and marketing approval from DCGI.
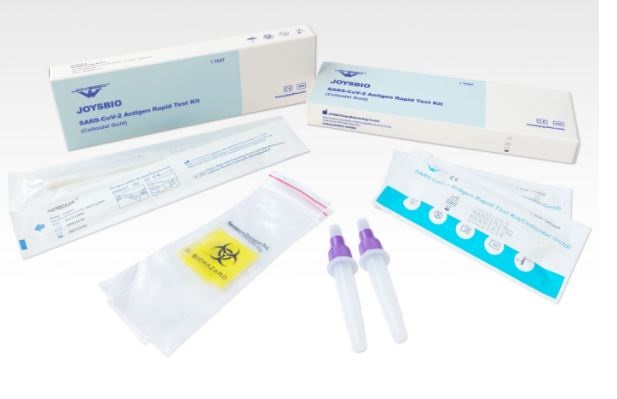
In June 2020, JOYSBIO Biotechnology proudly launched a brand new COVID-19 Antigen Rapid Test Kit (Colloidal Gold). The new coronavirus antigen test kit is a lateral movement immunoassay for the qualitative detection of SARS-COV-2 antigen (nucleocapsid protein) in higher respiratory samples with nasal swabs or saliva in the course of the acute part of an infection. An uncut sheet format is accessible.
Features
- 15-minute rapid detection
- Easy-to-operate coronavirus antigen test
- Less-invasive nasal (NS) swab pattern assortment
- CE-IVD marked
- Available in 1/2/5/20 checks/field.
Performance Characteristics
JOYSBIO’s coronavirus Ag test kit was independently evaluated at Centro Diagnostico Delta S.r.l. in Italy between October 2020 and January 2021. A complete of 107 constructive specimens had been examined with JOYSBIO’s COVID-19 Antigen Rapid Test Kit. These specimens had been collected from sufferers who’re suspected of COVID-19 with nasal swabs. The coronavirus antigen test kit’s sensitivity and specificity are in contrast in opposition to a CE-IVD marked RT-PCR test kit. This scientific analysis is carried out below the belief that SARS-CoV is now not spreading locally.
According to the scientific evaluation of 492 samples, the detection sensitivity is 98.13%, and the specificity is 99.22%.
- Positive Percent Agreement (PPA) = 105/107 (98.13%) (95%CI: 93.4%~99.8%)
- Negative Percent Agreement (NPA) = 382/385 (99.22%) (95%CI:97.7%~99.8%)
- Accuracy = (105+382)/492×100%=98.98%
- Kappa = 2×(105×382-3×2)/(108×385+107 ×384) = 0.97>0.5
The restrict of detection (LOD) of this product is 1.6 x 102 TCID50/mL, calculated by means of a gradient dilution technique.
COVID-19 Antigen Test Procedure
- Twist off the cap of the buffer bottle, rigorously dispense all buffer into the extraction tube。
- After accumulating higher respiratory pattern with nasal swab, insert the swab into the extraction tube, plunge the swab up and down within the fluid for no less than 10 seconds. Hold the swab in opposition to the underside of the tube, rotate three turns. DO NOT splash liquid out of the tube.
- Remove the swab whereas squeezing the edges of the tube to extract the liquid from the swab.
- Press the nozzle cap firmly onto the extraction tube. Mix totally by swirling or flicking the underside of the tube.
- Gently squeeze the tube’s inflexible physique, dispense two (2) drops of the buffer-specimen combination into the pattern effectively on the coronavirus antigen test cassette.
- Read the test outcomes between 15 and 20 minutes. Do not learn the outcomes after 20 minutes.
sars-cov-2-rapid-antigen-test
Key Points
- This interim steering is meant for healthcare suppliers who order antigen checks, obtain antigen test outcomes, or carry out point-of-care testing, in addition to for laboratory professionals who carry out antigen testing in a laboratory setting or on the level of care and report these outcomes.
- The goal of this interim technical steering is to help efficient scientific and public well being use of antigen checks for various testing conditions.
- This steering applies to all scientific and client makes use of of antigen checks and isn’t particular to any specific age group.
Abstract
Background
The Coronavirus illness 2019 (COVID-19) pandemic continues to unfold internationally. Hence, there’s an pressing want for rapid, easy, and correct checks to diagnose extreme acute respiratory syndrome coronavirus 2 (SARS-CoV-2) an infection. Performance traits of the rapid SARS-CoV-2 antigen detection test needs to be evaluated and in contrast with the gold customary real-time reverse transcription-polymerase chain response (RT-PCR) test for analysis of COVID-19 instances.
Methods
The rapid SARS-CoV-2 antigen detection test, Standard Q COVID-19 Ag kit (SD Biosensor®, Republic of Korea), was in contrast with the real-time RT-PCR test, Allplex 2019-nCoV Assay (Seegene®, Korea) for detection of SARS-CoV-2 in respiratory specimens. Four hundred fifty-four respiratory samples (primarily nasopharyngeal and throat swabs) had been obtained from COVID-19 suspected instances and make contact with people, together with pre-operative sufferers at Siriraj Hospital, Bangkok, Thailand throughout March–May 2020.
sars cov 2 antigen rapid test kit
Results
Of 454 respiratory samples, 60 (13.2%) had been constructive, and 394 (86.8%) had been unfavorable for SARS-CoV-2 RNA by real-time RT-PCR assay. The length from onset to laboratory test in COVID-19 suspected instances and make contact with people ranged from Zero to 14 days with a median of three days. The rapid SARS-CoV-2 antigen detection test’s sensitivity and specificity had been 98.33% (95% CI, 91.06–99.96%) and 98.73% (95% CI, 97.06–99.59%), respectively. One false unfavorable test outcome was from a pattern with a excessive real-time RT-PCR cycle threshold (Ct), whereas 5 false constructive test outcomes had been from specimens of pre-operative sufferers.
[Linking template=”default” type=”products” search=”antigen rapid test kit” header=”3″ limit=”19″ start=”1″ showCatalogNumber=”true” showSize=”true” showSupplier=”true” showPrice=”true” showDescription=”true” showAdditionalInformation=”true” showImage=”true” showSchemaMarkup=”true” imageWidth=”” imageHeight=””]