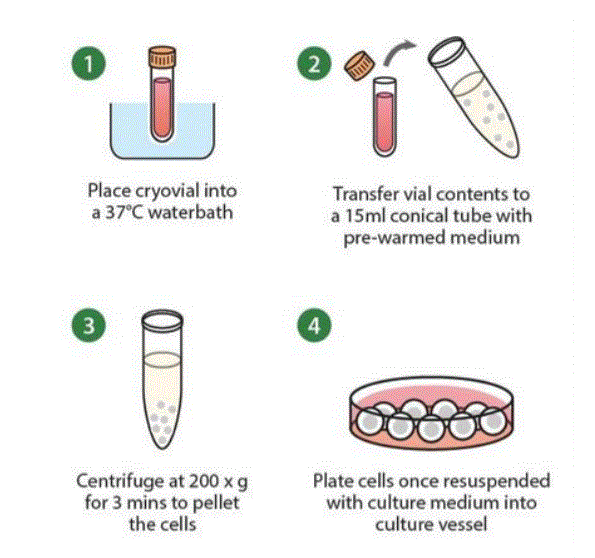
How to freeze thaw Cells?
- Remove the cryovial from the liquid nitrogen storage tank.
- Thaw the cells immediately by placing the lower half of the vial into a 37°C water tub
While agitating gently, eliminate after 60 seconds. Keep the cap out of the water to
prevent contamination. There should still be a few ice crystals made after thawing (it’s crucial not to over-thaw that the cryovials because the existence of DMSO is toxic to the
tissues ). - Decontaminate the vial by spraying and wiping the outside of the vial with 70%
ethanol. From that stage onwards, all operations should be rigorously carried out inside a
biological safety cabinet in aseptic conditions. - Gently re-suspend the cells in the vial and transfer the cell suspension to a 15 mL
sterile conical tube containing 5 mL of pre-warmed, complete media using a sterile
transfer pipette. 5. Centrifuge the cells in 200x gram for 3 minutes . Aspirate out the supernatant - Re-suspend the cell pellet in fresh, pre-warmed culture media and move the cells
Table
1 provides general guidelines to the volume of culture media needed for a Variety of
culture vessels.
Note: The magnitude of the culture vessel is exposed to this seeding density of the line (accessible
On the corresponding cell line page”seeding density” section). Otherwise, we recommend

Thawing entire cryopreserved content into T25 flask with instructed medium condition from
The propagation section of the product page.
- Incubate the culture at 37°C, 5% CO2, or another recommended culture environment
For the specific cell line. Incubate for 24 hours before processing cells for
downstream experiments.
| Culture Area (cm2) |
| 12- well plate 4 2.0 ml/ well |
| 6- well plate 9 3.0 ml/ well |
| T-25 flask 25 7.0 ml/ flask |
| T-75 flask 75 20.0 ml/ flask |
| 100 mm dish 55 10.0 ml/ dish |
| 150 mm dish 152 20.0 ml/ dish |
