Principle
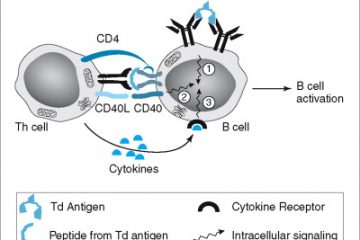
COVID-19 Antigen Detection Test is an immunoassay take a look at for fast and qualitative detection of SARS-CoV-2 an infection from swab specimens. Monoclonal anti-SARS-CoV-2 antibodies are coated on the take a look at line area. Antigens of SARS-CoV-2, if current within the specimens will work together with monoclonal antiSARS-CoV-2 antibodies conjugated with gold conjugate making antigen-antibodies conjugate advanced. This advanced migrates on the membrane by capillary motion, the place will probably be captured by the monoclonal anti-SARS-CoV-2 antibody coated on the take a look at line. A coloured take a look at line can be seen within the consequence window if SARS-CoV-2 antigens are current within the specimen. The depth of coloured take a look at line will differ relying upon the quantity of SARS-CoV-2 antigen current within the specimen.
SKU:NCTCAG-01
AVAILABILITY:Sold Out
SHIPMENT:1 or 2 Working Days for Free
SIZE:25 Tests/Kit
SENSITIVITY:96.6%
SPECIFICITY:100%
meriscreen-covid-19-antigen-test-kit-pack
MERISCREEN COVID-19 ANTIGEN TEST KIT
MeriScreen COVID-19 Antigen Detection Test is a fast immunochromatographic assay take a look at for the qualitative detection of SARS-CoV-2 antigen in nasopharyngeal swab pattern from a human physique.
- COATED WITH HIGHLY PURIFIED MONOCLONAL ANTI-SARS-COV-2 ANTIBODY
- RESULTS IN 20 MINUTES
- SAMPLE IS NASOPHARYNGEAL SWAB
- SENSITIVITY: 96.6%
- SPECIFICITY: 100%
Please Note:
Below listed kits are validated with the talked about batch quantity solely. Responsibility for batch to batch
consistency doesn’t lies with ICMR.
Minimum acceptance standards of sensitivity and specificity of Rapid Ag Test Kits:
Validated as a Point of Care Test (POCT) with out transport to a laboratory setupSensitivity: 50% and above
Specificity: 95% and above
Validated in a laboratory setup with samples collected in Viral Transport Medium (VTM)-
Sensitivity: 70% and above
Specificity: 99% and above
Antigen based mostly fast assessments that are US-FDA authorized can be utilized straight after due advertising and marketing approval from DCGI.
Brief methodology of use of COVID-19 Ag Respi-Strip (CorisBioConcept):
1. The Nasopharyngeal and/or Oropharyngeal swab will probably be collected from COVID-19 suspect affected person in Viral Transport Medium (VTM).
2. The collected swab in VTM will probably be delivered to the laboratory in acceptable chilly chain situations.
3. Once the pattern is delivered to the laboratory, it’s going to should be dealt with in a BSL-2 stage cupboard for aliquoting, placing in lysis buffer and loading the take a look at strip.
Steps 1 & 2 will probably be carried out as per the usual apply adopted for assortment and transport of samples for COVID-19 RT-PCR take a look at.
Step Three will should be carried out as per producers’ directions given with the take a look at equipment.
meriscreen-covid-19-antigen-test-kit
Easy to Use
User-friendly interface; Requires no particular experience or in depth coaching.
High Accuracy and Reliability
Excellent sensitivity and specifimetropolis in comparison with molecular and viral tradition strategies.
Convenient Storage Options
Small instrument dimension and room-temp storage appropriate for any workplace or lab.
Reduced User Errors
QR code scanning robotically identifies the take a look at kind
Dual Testing Options
Dual testing choices permit for higher testing flexibility
[Linking template=”default” type=”products” search=”MeriScreen Antigen Test” header=”3″ limit=”24″ start=”1″ showCatalogNumber=”true” showSize=”true” showSupplier=”true” showPrice=”true” showDescription=”true” showAdditionalInformation=”true” showImage=”true” showSchemaMarkup=”true” imageWidth=”” imageHeight=””]