Abstract
Infectious proteins (prions) are, mockingly, outlined by their resistance to proteolytic digestion. A defining attribute of the transmissible isoform of the prion protein (PrPSc) is its partial resistance to proteinase K (PK) digestion. Diagnosis of prion illness usually depends upon immunodetection of PK-digested PrPSc by Western blot, ELISA or immunohistochemical detection. PK digestion has additionally been used to detect variations in prion strains. Thus, PK has been an important device to detect and, thereby, management the unfold of prions.
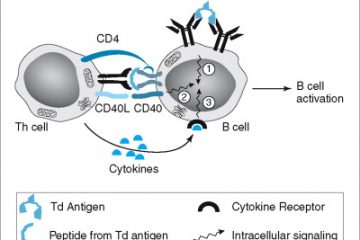
PK has additionally been used as a device to probe the construction of PrP. Mass spectrometry and antibodies have been used to establish PK cleavage websites in PrPSc. These outcomes have been used to establish the extra accessible, versatile stretches connecting the β-strand parts in PrPSc. These information, mixed with bodily constraints imposed by spectroscopic outcomes, had been used to suggest a qualitative mannequin for the construction of PrPSc. Assuming that PrPSc is a 4 rung β-solenoid, we have now threaded the PrP sequence to fulfill the PK proteolysis information and different experimental constraints.
Biological merchandise, similar to therapeutic proteins, monoclonal antibodies, vaccines, and cell and gene remedy merchandise have change into more and more essential. Unlike conventional chemically synthesized medication that act on a hydrophobic pocket of a protein to activate or inhibit a kinase pathway, biotherapeutics pharmacological motion is by way of binding to its goal or immunomodulation.
Due to the complicated nature of organic merchandise, scientists encounter a number of challenges from early drug discovery via improvement. For instance, throughout identification of a clone with the specified immunogenicity that may exhibit binding specificity to the goal and subsequent modulation or elicit the specified immune response, and additional throughout clone characterization, scale-up, and manufacturing of those merchandise.
From discovery via manufacturing, harnessing massive molecule medication to create protected and efficient therapies is complicated and costly. PerkinElmer allows scientists to develop and streamline their total biologics workflow to allow them to overcome these challenges and convey constant, high-quality, organic medicines to market quicker.
During the early phases of drug discovery, it’s crucial to know the goal biology, mechanism of motion (MoA) utilizing probably the most correct instrumentation together with automation, and software program to simplify complicated workflows from clone choice and expression to steer discovery and screening.
Accelerate your biomolecular discovery by utilizing a mixture of in vitro and in vivo methods accoupled with highly effective software program reworking information into insights.
- Cellular imaging for in vitro goal validation
- In vivo imaging for goal discovery
- Highly interactive visualization that facilitates the speedy isolation of compounds based mostly on key standards for additional evaluation
- In silico
Proteinase K, recombinant
Highlights
• Ready-to-use answer
• Active in a variety of response circumstances
Applications
• Isolation of genomic DNA from mouse tail
• Isolation of genomic DNA from cultured cells
• Removal of DNases and RNases when isolating DNA and RNA from tissues or cell strains
• Determination of enzyme localization
• Improving cloning effectivity of PCR merchandise
Note
• The beneficial working focus of Proteinase K is 0.05 to 1 mg/mL. The exercise of the enzyme is stimulated by 0.2 to 1% SDS or by 1 to Four M urea
• Ca2+ protects Proteinase K towards autolysis, will increase the thermal stability, and has a regulatory perform for the substrate binding website of Proteinase K
• Stable over a large pH vary: 4.Zero to 12.5, optimum pH 7.5 to eight.0
• Optimum exercise at 50 to55°C
• Rapid denaturation of enzyme happens at temperatures above 65°C