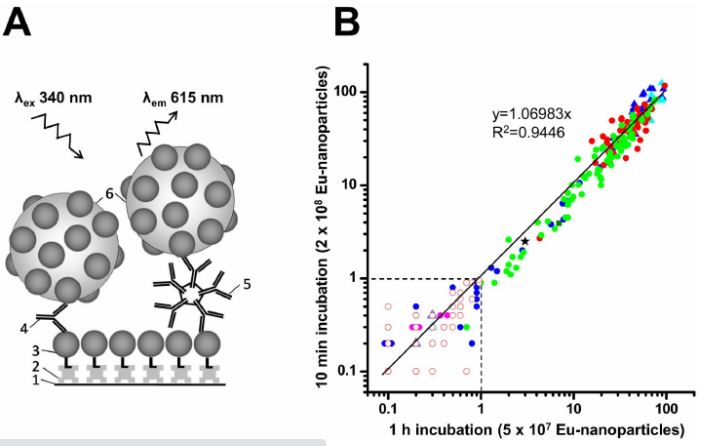
Assays carried out utilizing industrial kits as indicated. Results have been supplied by the panel provider. RPR, fast plasma reagin; ATA, anti-Treponema antibody; S/Co, sign to cutoff ratio; EIA, enzyme immunoassay; TPPA, Treponema pallidum particle agglutination assay; TPHA, Treponema pallidum haemagglutination assay; Neg, destructive; R, reactive; NR, non-reactive. RPR outcomes are endpoint dilutions. S/Co ratios ≥1.Zero are thought of reactive.
b Values point out S/Co ratios obtained on this examine, utilizing the in-house TRF immunoassays with 10 min and 1 h incubation instances. Samples with S/Co values <1.Zero are designated as destructive (−) and people with values ≥1.Zero are designated as optimistic (+).
Abstract
A newly developed immunochromatography assay, DainaScreen TPAb (Dainabot, Tokyo), to detect antibodies particular to Treponema pallidum was evaluated. When we examined serum and plasma samples of Syphilis Mixed Titer Performance Panel PSS201 (Boston Biomedica, Inc. , Bridgewater, MA, U.S.A.), all of the check outcomes obtained by DainaScreen TPAb have been similar to these decided by fluorescent treponemal antibody absorption check (FTA-ABS).
Both within-run and day-to-day variation checks have been extremely exact, and no discrepant interpretation was obtained by the totally different medical technicians carried out. Also, the testings of complete blood and plasma for particular person samples gave identical interpretations. The minimal detectable antibody titer was equal to that of Mediace TPLA (Sekisui Chemicals, Osaka) decided by Behring Nephelometer Analyzer (Dade Behring, Marburg, Germany). All the check outcomes by DainaScreen TPAb for scientific serum samples have been similar to these by Mediace TPLA. With these outcomes, we are able to conclude that DainaScreen TPAb is a fast, sensible and easy-to-perform different to detect antibodies particular to Treponema pallidum, particularly as being a point-of-care testing.
syphilis-mixed-titer-performance-panel
Methods
Using Brazilian Ministry of Health information on ladies identified with maternal syphilis between January 1, 2010, and December 31, 2018, we carried out a random-effects logistic regression mannequin with a cluster correction on the state stage to judge predictive elements of penicillin therapy.
Results
We noticed yearly will increase in circumstances of pregnant ladies with syphilis from 2010 to 2018. There was vital variation by state: 52,451 circumstances have been reported in São Paulo, adopted by 26,838 in Rio de Janeiro. Among 215,937 circumstances of maternal syphilis, 91·3% acquired penicillin. In the random-effects mannequin, a non-treponemal titer ≥1:16 was related to 1·44 larger odds of receiving penicillin (95% confidence interval [CI]: 1·391·48), and prenatal care was related to a 2·12 elevated odds of receiving penicillin (95% CI: 2·022·21). Although there may be an affiliation between the absence of prenatal care and insufficient therapy for syphilis, 83·2% of girls on this cohort who didn’t obtain penicillin have been engaged in prenatal care.
Conclusions
Providers could inappropriately exclude low non-treponemal titers and thereby fail to make use of penicillin therapy in maternal syphilis. While the reason for the maternal syphilis epidemic in Brazil is multifactorial, we imagine our findings can be utilized to develop focused interventions all through Brazil in addition to form public well being initiatives globally.
Description
- Characterized samples and complete information are supplied for comparative evaluation.
- No preservatives have been added
- 1 vial per member, 17 members, 0.5mL per vial
[Linking template=”default” type=”products” search=” Mixed Titer Performance Panel” header=”2″ limit=”25″ start=”1″ showCatalogNumber=”true” showSize=”true” showSupplier=”true” showPrice=”true” showDescription=”true” showAdditionalInformation=”true” showImage=”true” showSchemaMarkup=”true” imageWidth=”” imageHeight=””]